Glaukos Avedro
Cross-linking
The only therapy of
keratoconus approved
by the FDA
(United States Food and Drug Administration)
The cross-linking procedure patented by
Glaukos Avedro is the first and only therapy approved by
United States Food and Drug Administration
which strengthens the cornea and slows the progression of keratoconus
Keratoconus is a condition in which the normal spherical shape of the cornea progressively thins and weakens, causing a cone-shaped deformation.
Questa deformazione può causare un severo peggioramento della vista e nei casi più avanzati l'assottigliamento progressivo della cornea può rendere necessario un trapianto di cornea.
Questa deformazione può causare un severo peggioramento della vista e nei casi più avanzati l'assottigliamento progressivo della cornea può rendere necessario un trapianto di cornea.
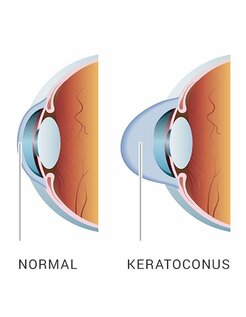
Keratoconus in the absence of adequate treatment
can cause severe vision loss
and may require multiple corneal transplants,
seriously threatening the quality of life of patients


Il cross-linking corneale è una procedura mini-invasiva che unisce l'uso di eye drops with Vitamin B2 and an ultraviolet system to stop the progression of keratoconus.
- It creates new cross-links between the collagen fibers that make up the cornea
- The fibrils shorten and become thicker
- The cornea becomes stiffer and firmer

Nell'Aprile 2016 la FDA degli Stati Uniti ha approvato Photrexa® Viscous, Photrexa® e il sistema KXL® per il cross-linking corneale, una procedura mini-invasiva che combina l'uso di gocce oculari con Vitamina B2 e luce ultra-violetta (UV)
Al momento Glaukos Avedro è l'unica terapia per il cheratocono ad avere ottenuto questa importante certificazione
Glaukos Avedro cross-linking is available at
Aci Castello - Doctors Zagari
Bologna - Dr. Muraca Perrone
Bologna-Sant’Orsola - Prof. Luigi Fontana
Brescia - Eyecare eye centre
Cagliari - Dr. Paolo Tronci
Campobasso - Dr. Paolo Polisena
Castel San Pietro Terme - Dr. Rocco De Fazio
Catania - Dott. Francesco Famà
Catanzaro - Dr. Miguel Rechichi
Conegliano Veneto - Dr. Giovanni Prosdocimo
Correggio - Dr Marco Vecchi
Cosenza - Dr. Miguel Rechichi
Cuneo - Dr. Guido Caramello
Domodossola - Dr. Romolo Protti
Genoa - Health Ultralaser
Lugano - Dr. Lorenzo Artaria
Mascalucia - Dr. Salvatore Lo Grasso
Mestre - Dr. Dario Surace
Milan - Dr. Francesco Carones
Milan - Quattroelle eye studio
Modena - Dr. Paola Benedetti
Modena - Dr. Gianmaria Cavallini
Modena - Dr. Federico Nizzola
Morrovalle - Dr. Vincenzo Ramovecchi
Naples - Day Surgery Center
Naples - Dr. Alfredo Venosa
Naples - VanvitelliI University Hospital
Padua - Dr Paolucci Pierpaolo
Parma - Dr. Claudio Macaluso
Pavia - Dr. Roberto Ceccuzzi
Piacenza - Dr. Luigi Lodigiani
Pisa - Dr. Marco Fantozzi
Reggio Calabria - Dr. Demetrio Spinelli
Rome - Prof. Aldo Caporossi
Rome - Gemelli Hospital
Rome - Dr. Luca Gualdi
Rovigo - Dr. Luigi Caretti
San Benedetto del Tronto - Dr. Luca Cesari
San Severino Marche - Dr. Vincenzo Ramovecchi
Sassari - Dr. Paola Piccinini
Selargius - Dr. Miguel Rechichi
Siena - Prof. Cosimo Mazzotta
Siena - Prof. Aldo Caporossi
I want to receive more information about Glaukos Avedro

KXL is produced by the leading American cross-linking company Glaukos Avedro.
https://glaukos.com/
Page intended for healthcare professionals.
In compliance with the provisions provided by the Ministry of Health regarding health advertising relating to medical devices, in vitro diagnostic medical devices and medical-surgical devices, we inform you that the contents of this page are intended exclusively for healthcare professionals. Where present, the description of the products is for informational purposes only and is not intended to promote them.
